Alyra Biotech is a bio-pharmaceutical company with novel, patented, first-in-class, neuroimmune-modifying, intrauterine products to treat neuroimmune conditions including pain, currently in human trials.
We modulate the Uterus-Brain Axis to reduce pelvic pain, bowel and bladder symptoms, headache, fatigue, endometriosis-associated pain and premenstrual syndrome symptoms.
Alyra Biotech is guided by our close collaboration with consumers and our in-house end user research. Wellbeing and the human experience are central of our innovation process.
Alyra Biotech Corporate Highlights
Committed to improving the management of pain and wellbeing in women
Patents granted in US, China, Australia. Additional patents pending.
Products driven by patient experience, and backed by novel scientific innovation
Expert management team with proven development & commercial success in women’s health
The Uterus-Brain Axis
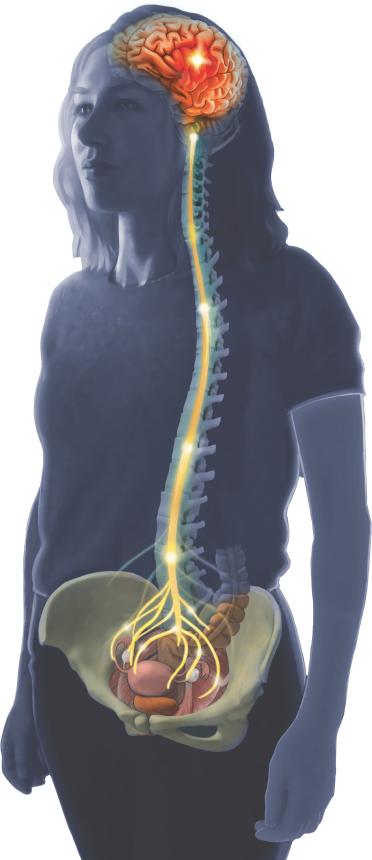
Most of us have heard about the Gut-Brain Axis: the way activation of the immune system in our bowel talks to our brain and influences how we feel.
The Uterus-Brain Axis works in a similar way, yet is under-researched as a cause of pain and symptoms in women.
Some women have excess activation of the immune system in their uterus. Immune activation spreads via nerves, circulating immune cells and chemicals called cytokines to the brain and spinal cord. Glia (immune cells) in the central nervous system become activated and alter the way nerves in the brain function. This causes symptoms that can include pain, fatigue, mood disorders, bowel symptoms or poor sleep. This explains the wide range of symptoms from inside and outside the pelvis reported by women with period pain (1)
Immune activation in the Uterus involves activation of immune Toll-Like Receptor 4 (TLR4), an immune receptor on the surface of cells. These receptors are usually activated by bacteria, but they are also activated by the breakdown of tissue and the presence of blood. Tissue breakdown, blood and sometimes bacteria are all present during menstruation, a time of maximal pain and symptoms.
Communication between the Uterus and Brain
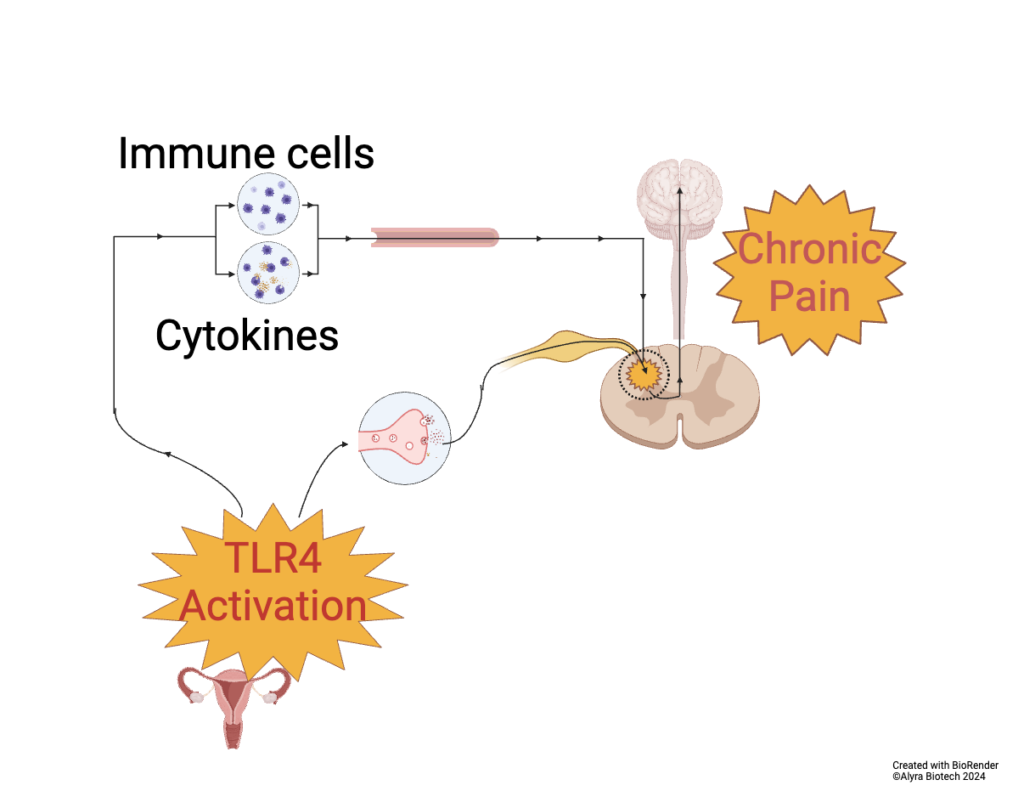
Women with pre-existing period or pelvic pain have a primed immune system. When an intrauterine device is inserted, inflammation in the uterus increases, the immune system activates, and symptoms can worsen.
Alyra Biotech’s Lead Device adds a second drug to the IUD to reduce immune activation, reduce pain, and allow more women to successfully use an IUD.
Additional devices across a range of medical indications are envisaged.
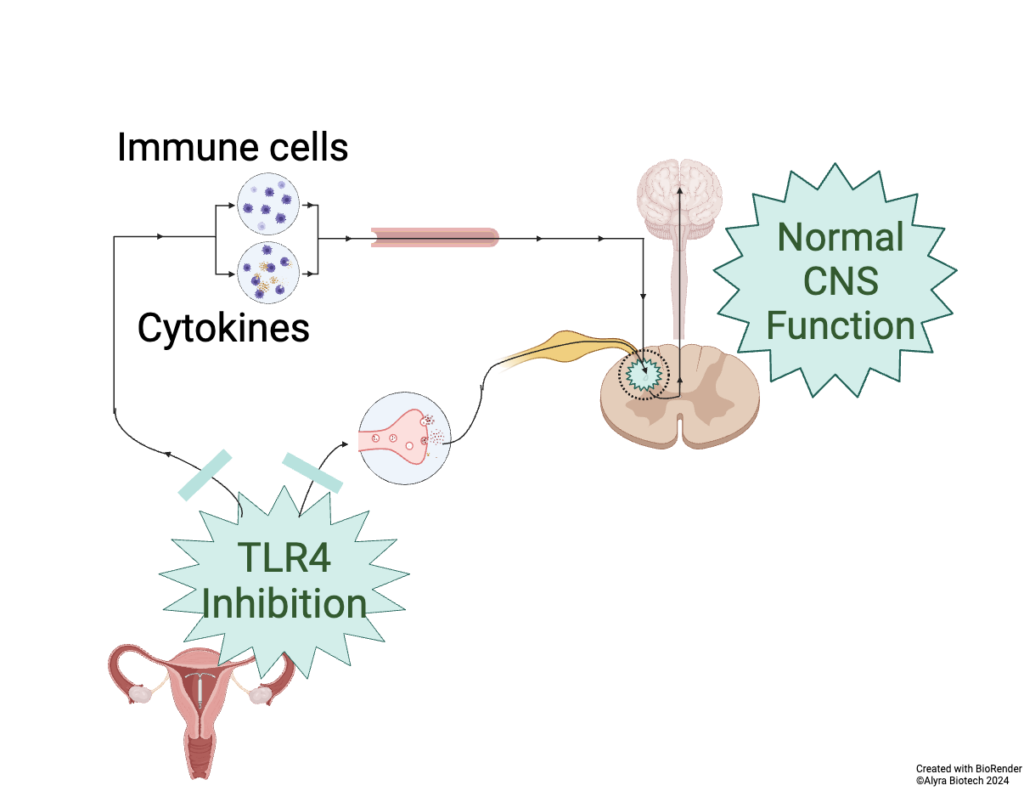
Product development at Alyra Biotech is de-risked by using drugs with known efficacy and long-term safety data in humans.
Targeted intrauterine release allows low dose, sustained drug release, with low risk of systemic side effects.
Alyra’s first-in-human trial has already confirmed a suitable drug release profile from the device, drug levels in blood below the level associated with adverse effects, and high tolerability.